Assessing Vascular Inflammation and Safety with the Perfusable 3D OrganoReady® Blood Vessel HUVEC
.webp)

Why This Is Important

Challenges

Need
MIMETAS’ Answer
.svg)
Organ Model
.svg)
Features
• Stable, functional and leak tight endothelial barrier
• Reproducible inflammatory activation
• Robust monocyte adhesion assays
• Reliable toxicity read-outs for screening
.svg)
Offering
Custom CRO Services
Studying Vascular Inflammation
Vascular inflammation and monocyte adhesion are key drivers in many diseases. Physiologically-relevant endothelial models areessential to evaluate inflammatory pathways, drug responses, and vasculartoxicity.
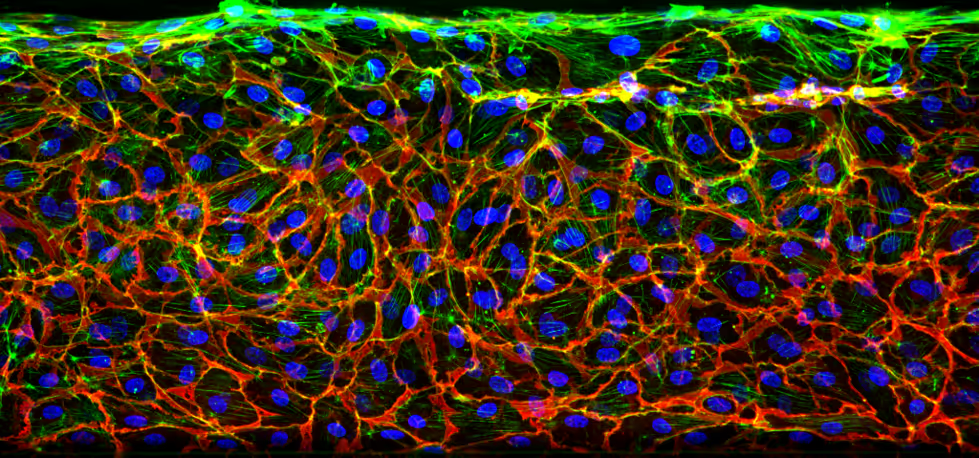
Characterization of the Endothelial Vessel
High-resolution imaging of the OrganoReady Blood Vessel HUVEC reveals a well-organized endothelial structure, marked by continuous VE-cadherin junctions and aligned actin fibers along the tubular wall. This architecture reflects a stable, cohesive barrier in the 3D perfusable vessel (figure 1).
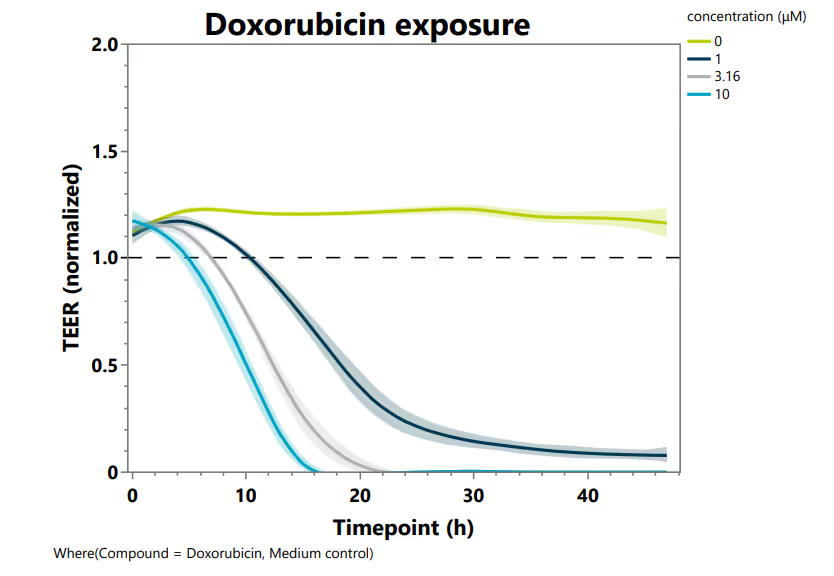
Assessing Inflammation and Toxicity
The model delivers a strong dose-dependent toxicity profile after doxorubicin treatment, captured through real-time transepithelial/transendothelial electrical resistance (TEER) measurement (Figure 2A). Cytokine treatment also triggers a significant, dose-dependent upregulation of ICAM-1, confirming robust and physiologically relevant endothelial activation (Figure 2B).
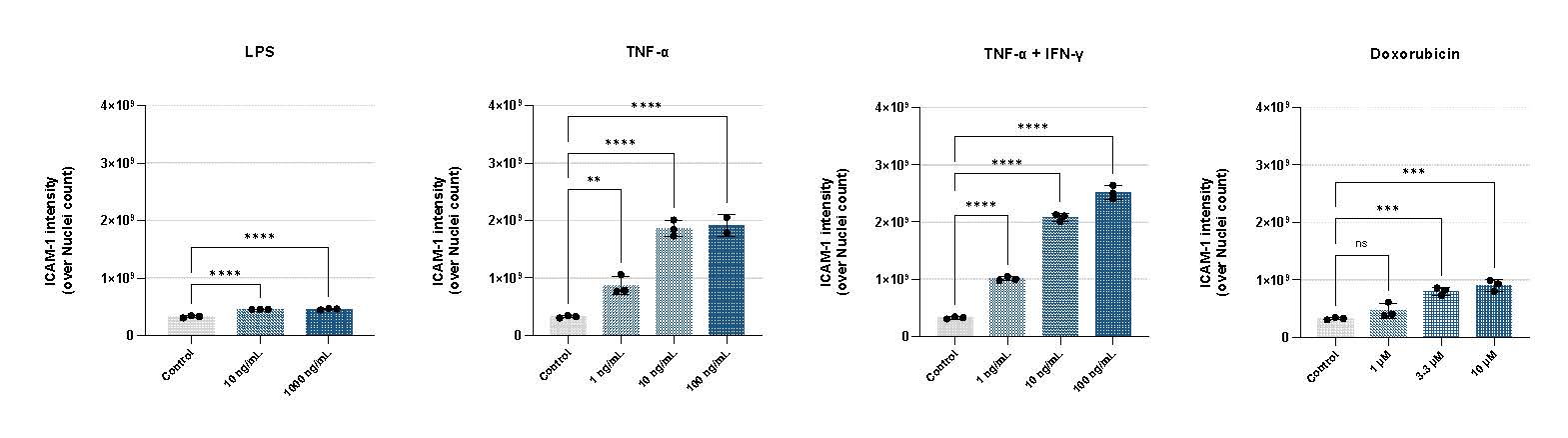
Robust Monocyte Adhesion Assay
The model supports perfusion with peripheral blood mononuclear cells (PBMCs) to study adhesion under inflammatory conditions (Figure 3). TNF-α stimulation produces a marked increase in monocyte attachment, demonstrating that OrganoReady HUVEC can effectively capture key inflammation-driven cellular interactions.

Summary
- Physiologically Relevant Vascular Inflammation: A perfusable 3D human blood vessel model with a stable, leak-tight endothelial barrier enables robust modeling of inflammatory activation, barrier disruption, and monocyte adhesion under flow.
- Predictive Safety and Toxicity Assessment: Real-time TEER measurements and dose-dependent endothelial responses provide sensitive, human-relevant readouts for vascular toxicity and drug-induced inflammation.
- Translational Immune–Endothelium Interactions: Controlled cytokine stimulation and PBMC perfusion support reproducible assessment of ICAM-1 upregulation and immune cell adhesion, bridging mechanistic insight with screening-ready throughput.
References
- Henein, M. Y., Vancheri, S.,Longo, G., & Vancheri, F. (2022). The role of inflammation incardiovascular disease. International journal of molecular sciences, 23(21),12906.
- Nam, U., Lee, S., Ahmad, A., Yi, H. G., &Jeon, J. S. (2024). Microphysiological systems as organ-specific in vitrovascular models for disease modeling. BioChip Journal, 18(3), 345-356.
Selected Resources
.avif)


.avif)



