Inflamed Gut Models for Studying Drug Response in Inflammatory Bowel Disease


Why This Is Important

Challenges

Need
MIMETAS’ Answer
.svg)
Organ Model
.svg)
Features
.svg)
Offering
IBD-on-a-Chip
While IBD’s multifactorial pathology creates complexity, each aspect represents a valid therapeutic angle and is being explored by pharmaceutical companies to varying degrees. These include an altered microbiome, damaged epithelial barriers, reduced mucus formation, stromal activation and cytokine release, increased immune cell infiltration, hypervascularization, impaired stem cell renewal and differentiation. To capture these multitude of factors, understand their interplay, and elucidate the causality of events, requires advanced models that capture tissue organization in a more comprehensive manner.
Inflamed Gut Models for Studying Drug Response in Inflammatory Bowel Disease
MIMETAS captures the complexity of the intestine by combining components such as intestine epithelium, stroma, vasculature and immune cells in perfused 3D models and assays. MIMETAS scientists utilize adult stem cell-derived colon organoids from primary patient material, which retain donor-specifics such as genetic profiles, cell differentiation patterns, and responses to stimuli2-4.
Within the OrganoPlate platform, MIMETAS scientists create colon organoid tubules containing goblet cells, enterocytes, and proliferating cells. The models are further complemented by inclusion of endothelial cells, fibroblasts, and/or macrophages (Figure 1). While not shown in the data here, macrophages can also be isolated from the same patient if necessary.
These organoids exhibit a robust barrier function over time, both in monoculture and co-culture, while even enhanced in a tri-culture with fibroblasts and macrophages (Figure 2). The tri-culture also demonstrates increased resistance to Afatinib, a compound known to induce epithelial damage (Figure 3), highlighting its potential for testing drug toxicity.

Exploring Macrophage Plasticity
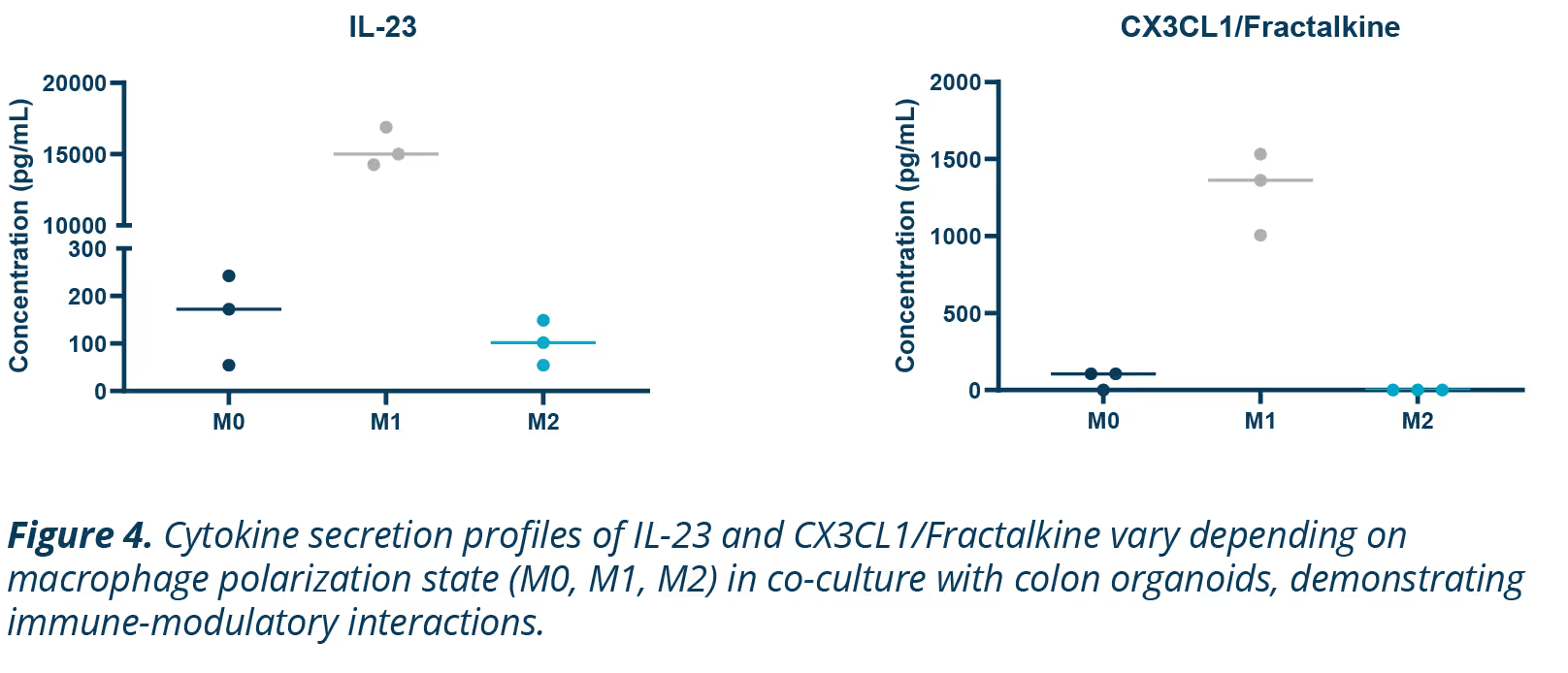
Macrophages are key regulators of immune responses, displaying distinct behavior depending on their phenotype. M1 macrophages are proinflammatory, driving inflammation, while M2 macrophages are anti-inflammatory, promoting tissue repair.
This phenotypic flexibility makes them interesting targets for studying immune interactions and inflammatory diseases like IBD (Figure 4).
Summary
- Comprehensive Gut Models: Perfused colon tubules derived from adult stem cell organoids in co-culture with fibroblasts and macrophages have enhanced barrier function and effectively recapitulate gut stromal-immune interactions.
- Accurate Immune Modeling: Macrophage plasticity is utilized to include macrophages proinflammatory and immune suppressive phenotypes, providing a valuable tool for studying IBD and testing therapies.
References
- Taku Kobayashi, Toshifumi Hibi. "Improving IBD outcomes in the era of many treatment options". Nature Reviews Gastroenterology & Hepatology 2023; 20:79-80
- Nick Barker, Marc van de Wetering, Hans Clevers. "The intestinal stem cell". Genes & Development 2008; 22:1856-1864.
- Hans Clevers, Ryan K. Conder, Vivian S.W. Li, Matthias P. Lutolf, Ludovic Vallier, Sarah Chan, Tracy C. Grikscheit, Kim B. Jensen, Paolo De Coppi. "Tissue-Engineering the Intestine: The Trials before the Trials". Cell Stem Cell 2019; 24:855-859
- Kim E Boonekamp, Talya L Dayton, Hans Clevers. "Intestinal organoids as tools for enriching and studying specific and rare cell types: advances and future directions". Journal of Molecular Cell Biology 2020; 12:562-568.


.jpg)


.png)

.webp)