OrganoReady® Colon Organoid
Delivered as perfusable, leak-tight tubules with a functional intestinal barrier.
The OrganoReady® Colon Organoid is an adult stem cell–derived, ready-to-use HUB colon organoid model delivered as perfusable, leak-tight tubules with a functional intestinal barrier. This assay-ready format enables accurate and reproducible gastrointestinal (GI) toxicity testing, addressing a critical need in preclinical drug development.

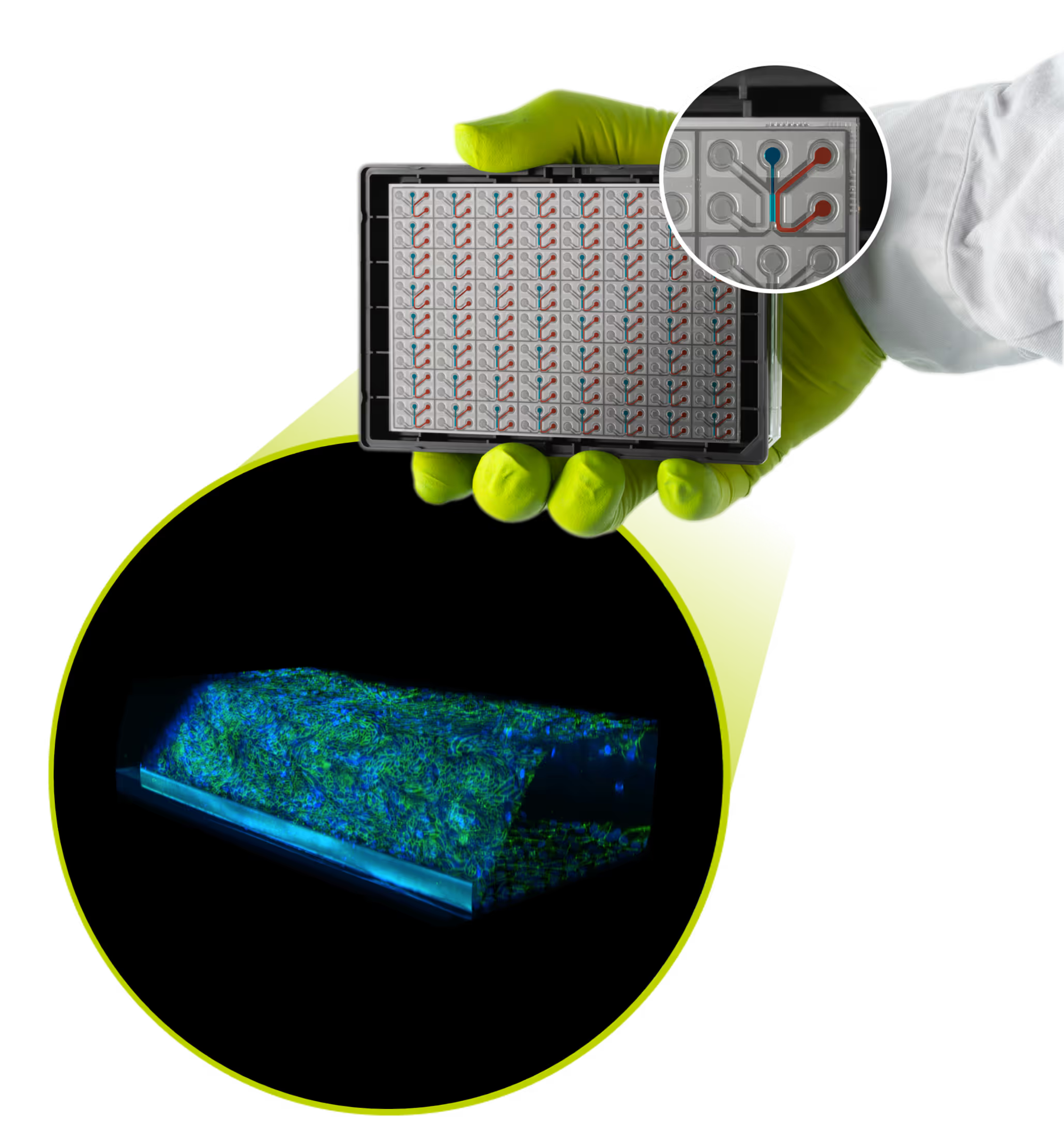
Specifications
Each OrganoReady Colon Organoid comes pre-seeded with 40 or 64 ready-to-use colon organoids tubules grown against validated Collagen-I with stable barriers.
Plate type: OrganoPlate® 3-lane 40 or 64
Configuration: Colon organoid tubules against Collagen-1
HUB license (single-use) included
Access: Apical and basal
Recovery time: Functional barrier re-forms within a few days post-arrival; no additional seeding or preparation required
Intestinal barrier integrity
GI toxicity
Inflammation
Nutrient and metabolite transport
Food safety
Pre-biotics and microbiome interactions
Applications
The OrganoReady Colon Organoid model replicates key features of the human intestinal tissue, enabling a range of disease-relevant and preclinical assays. All applications benefit from our OrganoTEER® system for barrier function measurement.
Validation
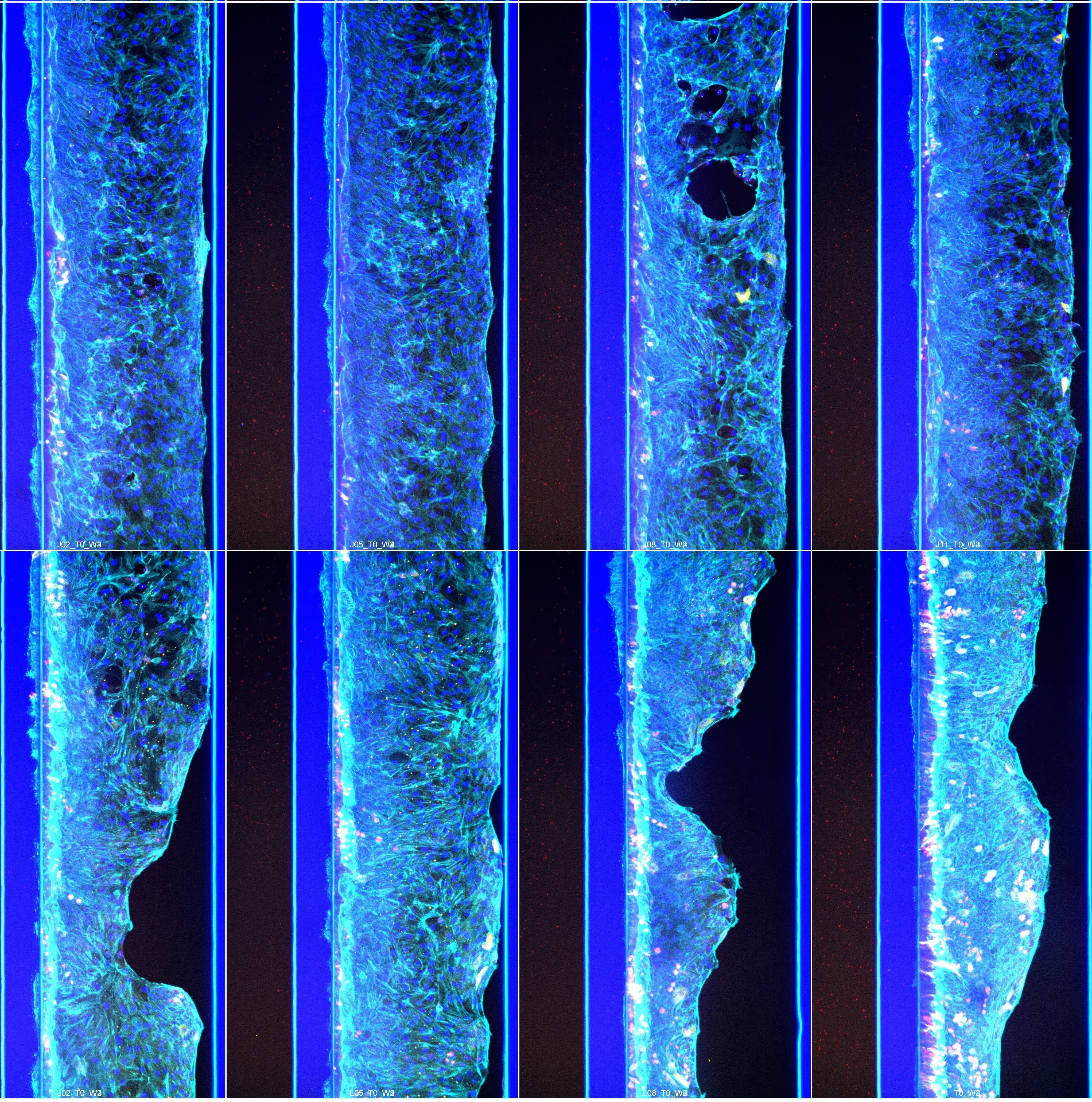
The OrganoReady Colon Organoid model demonstrates adult intestinal tissue characteristics, including a mature epithelial phenotype with apical–basolateral polarity, functional transporter activity, and proliferative profile.

Organoid tubules comprise all major gut cell subtypes, including enterocytes, goblet cells, proliferative cells, and stem cells
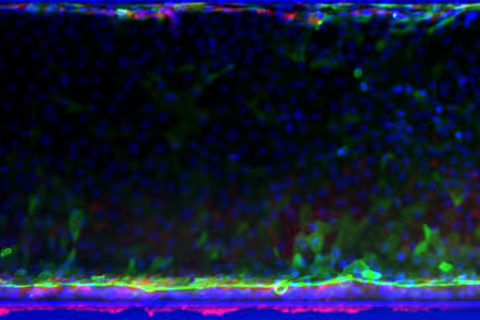
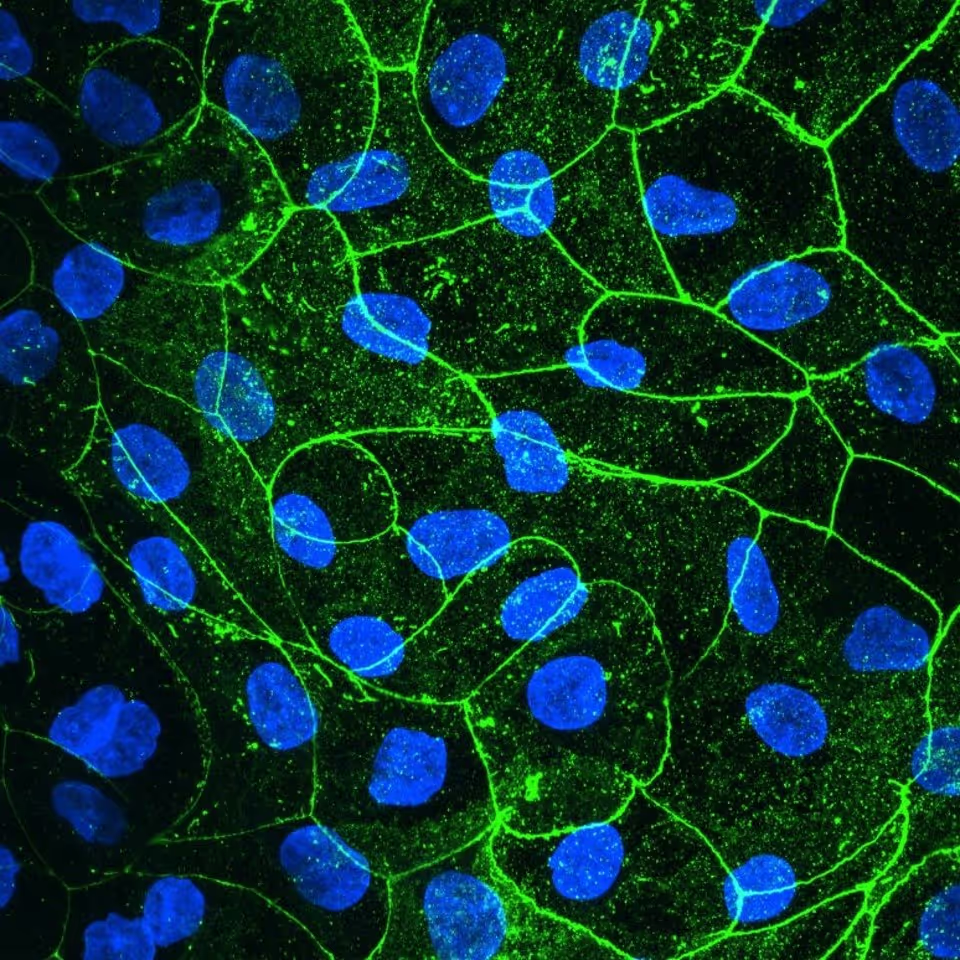
Colon organoids are fully polarised with apical brush borders, BCRP, and PgP transporter expression and basal Integrin β4
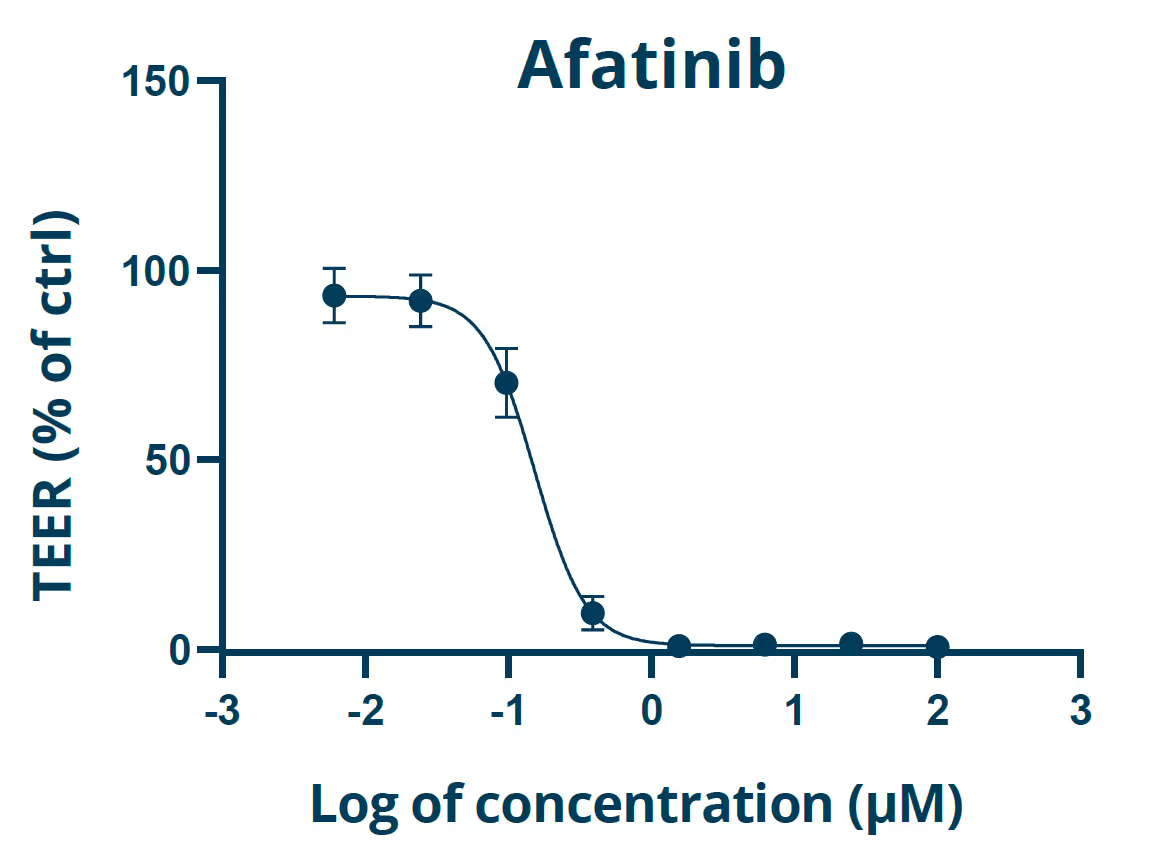
Long-term viability and reproducible barrier function (TEER) that can be modulated with toxicants
Resources

How to Access Our OrganoReady® Colon Organoid
Services
MIMETAS provides tailored, fee-for-service solutions to support therapy selection and risk assessment. Using the OrganoReady® Colon Organoid model, we offer scalable assays for disease modeling, compound prioritization and safety, as well as DMPK ranking.
Shipped Product
The OrganoReady® Colon Organoid is offered as a ready-to-use model shipped directly to your lab, complete with culture media and optimized protocols. After a short recovery, the perfusable tubules are assay-ready, ideal for evaluating gut physiology and gastrointestinal ADME/toxicity studies.
