Modeling Glioblastoma's Tumor Microenvironment: Blood-Brain Barrier Interactions and Vascular Dynamics
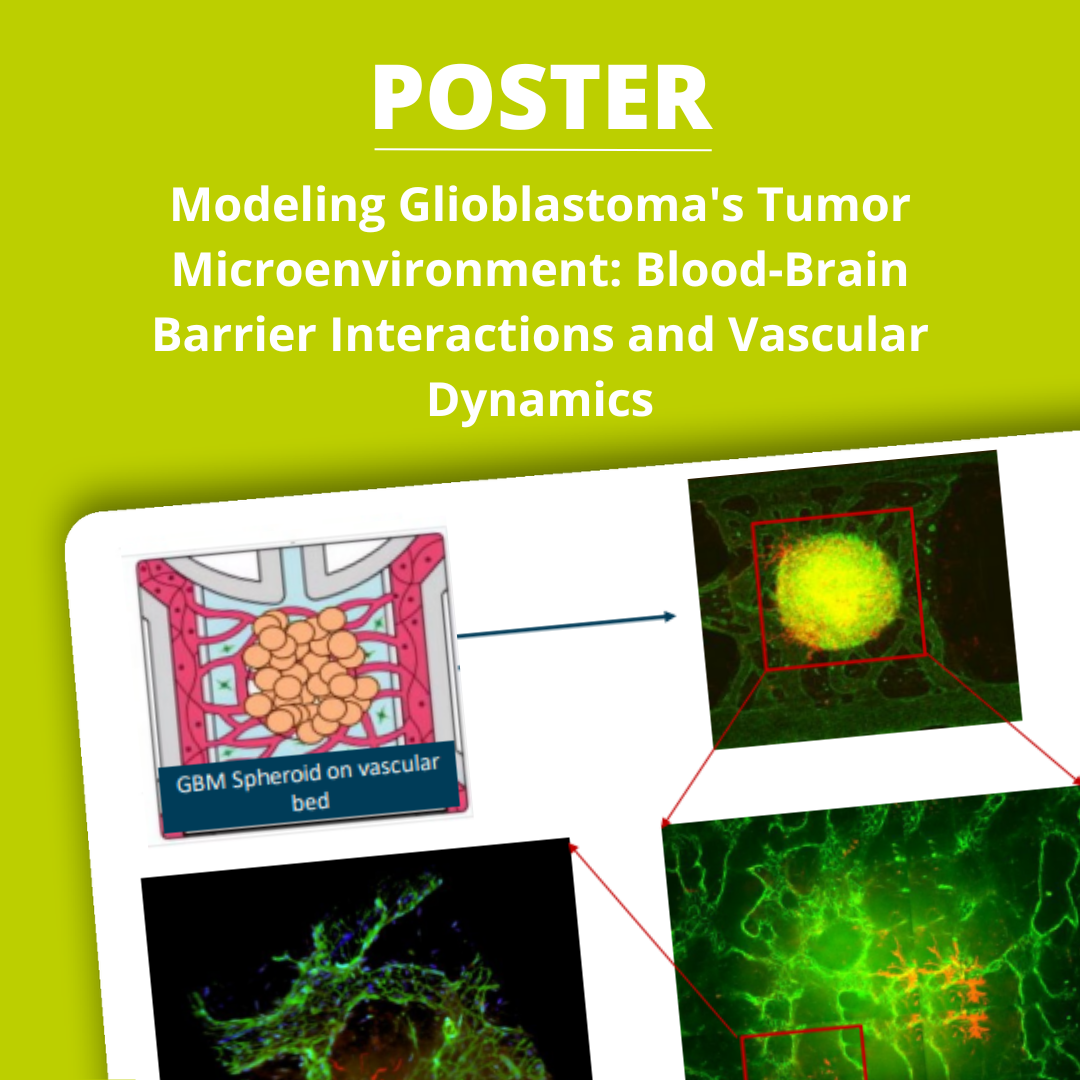
Understanding how glioblastoma (GBM) disrupts the blood-brain barrier (BBB) and reshapes the vascular landscape is essential to improving therapeutic strategies for this highly aggressive brain tumor. Our study presents a multi-model approach to uncover how GBM affects BBB integrity, vascular perfusion, and immune interactions, using both vascularized GBM-on-a-chip systems and patient-derived tumor spheroids.These advanced models replicate key features of the human brain tumor microenvironment, enabling researchers to explore GBM-driven barrier disruption, non-perfusable vessel formation, and differential cytokine signaling across GBM cell lines.Key Features of the ModelsBlood-brain barrier chip for analyzing GBM-induced barrier disruptionVascularized GBM model incorporating endothelial cells, pericytes, astrocytes, and GBMQuantification of angiogenic factor release and vascular structure changesPatient-derived spheroid integration on preformed vasculature
Modeling Glioblastoma's Tumor Microenvironment: Blood-Brain Barrier Interactions and Vascular Dynamics

Modeling Glioblastoma's Tumor Microenvironment: Blood-Brain Barrier Interactions and Vascular Dynamics
A multi-model GBM-on-a-chip platform replicating the human tumor microenvironment to study how glioblastoma disrupts the blood-brain barrier, alters vascular dynamics, and modulates immune signaling using patient-derived spheroids and vascularized systems.

Modeling Glioblastoma's Tumor Microenvironment: Blood-Brain Barrier Interactions and Vascular Dynamics
A multi-model GBM-on-a-chip platform replicating the human tumor microenvironment to study how glioblastoma disrupts the blood-brain barrier, alters vascular dynamics, and modulates immune signaling using patient-derived spheroids and vascularized systems.

Modeling Glioblastoma's Tumor Microenvironment: Blood-Brain Barrier Interactions and Vascular Dynamics
A multi-model GBM-on-a-chip platform replicating the human tumor microenvironment to study how glioblastoma disrupts the blood-brain barrier, alters vascular dynamics, and modulates immune signaling using patient-derived spheroids and vascularized systems.

Modeling Glioblastoma's Tumor Microenvironment: Blood-Brain Barrier Interactions and Vascular Dynamics
A multi-model GBM-on-a-chip platform replicating the human tumor microenvironment to study how glioblastoma disrupts the blood-brain barrier, alters vascular dynamics, and modulates immune signaling using patient-derived spheroids and vascularized systems.

Modeling Glioblastoma's Tumor Microenvironment: Blood-Brain Barrier Interactions and Vascular Dynamics
A multi-model GBM-on-a-chip platform replicating the human tumor microenvironment to study how glioblastoma disrupts the blood-brain barrier, alters vascular dynamics, and modulates immune signaling using patient-derived spheroids and vascularized systems.

Modeling Glioblastoma's Tumor Microenvironment: Blood-Brain Barrier Interactions and Vascular Dynamics
A multi-model GBM-on-a-chip platform replicating the human tumor microenvironment to study how glioblastoma disrupts the blood-brain barrier, alters vascular dynamics, and modulates immune signaling using patient-derived spheroids and vascularized systems.

Modeling Glioblastoma's Tumor Microenvironment: Blood-Brain Barrier Interactions and Vascular Dynamics
A multi-model GBM-on-a-chip platform replicating the human tumor microenvironment to study how glioblastoma disrupts the blood-brain barrier, alters vascular dynamics, and modulates immune signaling using patient-derived spheroids and vascularized systems.

Modeling Glioblastoma's Tumor Microenvironment: Blood-Brain Barrier Interactions and Vascular Dynamics
A multi-model GBM-on-a-chip platform replicating the human tumor microenvironment to study how glioblastoma disrupts the blood-brain barrier, alters vascular dynamics, and modulates immune signaling using patient-derived spheroids and vascularized systems.

