About the research
One of the major challenges for the development of successful therapeutics for chronic kidney disease lies in the ability to effectively establish 3D models that can mimic the complex structure and function of the glomerular filtration barrier.
The unmet needs in nephrology
In the majority of the current glomerular models, podocytes and glomerular endothelial cells are separated by a synthetic membrane with openings (pores) that allow free exchange of media and growth factors. These membranes, however, do not allow the proper crosstalk between glomerular cells which is key for glomerular filtration barrier function.
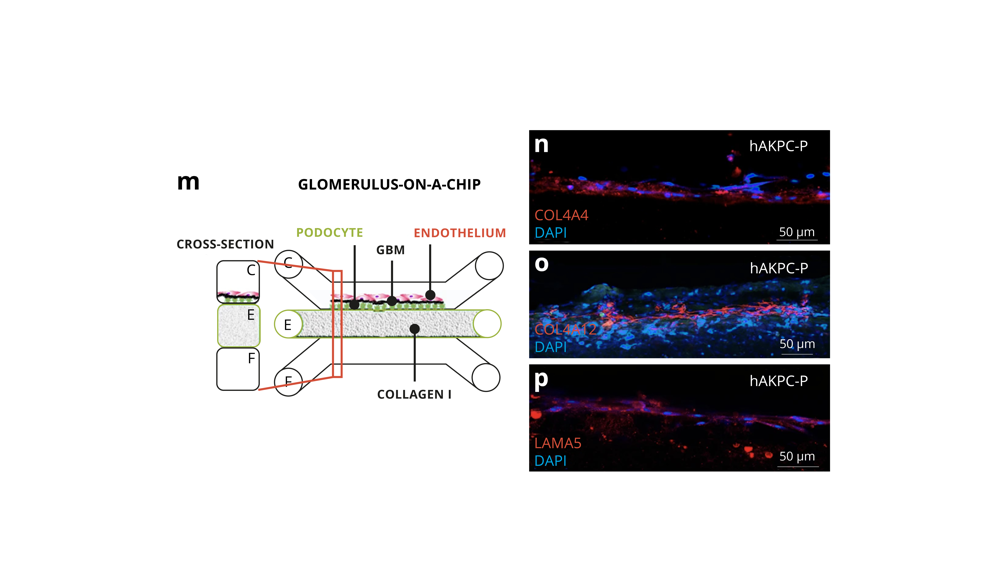
To enable proper crosstalk, researchers from Children’s Hospital Los Angeles (CHLA) developed a glomerulus-on-a-chip model composed of human podocytes and human glomerular endothelial cells in the OrganoPlate® platform, which enables co-culturing of these two cell types, without artificial membranes.
Glomerulus-on-a-chip
The researchers from CHLA showed that the developed glomerulus-on-a-chip is similar in function and structure to the glomerulus, including permselectivity. Furthermore, by exposing the model to sera from patients with anti-podocyte autoantibodies the chips show albuminuria proportional to patients’ proteinuria, a phenomenon that was not observed with sera from healthy controls or individuals with primary podocyte defects.
Model for drug screening
The developed in vitro glomerulus model of CHLA is a unique model for the screening of drugs targeting the glomerular filtration barrier, as it responds to human nephrotoxic serum and nephroprotective treatment similar to the in vivo human glomerulus. It also proved to be relevant for renal disease modeling and drug testing. During this study, a total of 2000 independent chips (50 OrganoPlates) were analyzed. The high number of analyzed chips supports the validation of this model for high-throughput screening of therapeutic compounds.
Key results
- A unique model for the screening of drugs targeting the glomerular filtration barrier
- Deposition observed of de novo extracellular membrane components that are specific to the glomerulus
- The model shows its potential for high-throughput screening of therapeutic compounds. A total of 2000 independent chips, 50 OrganoPlates, were analyzed.
Want to know more?
Get up to speed with 3D tissue culture and learn how OrganoPlate® supports your research needs.
