About the research
Intestinal inflammation is among the most prevalent health indications in the Western world. It comprises a range of diseases, from the less defined irritable bowel syndrome to full impairment of intestinal function in inflammatory bowel disease (IBD).
The development of preventative and therapeutic agents proved to be challenging as many of the processes involved in intestinal inflammation are still poorly understood. Reliable, robust, and high-throughput in vitro models that capture key aspects of intestinal inflammation are therefore essential for the drug development process but are still lacking. Additionally, each stage of the drug development process comes with its own specific demands in terms of complexity and screenability of the model, which further emphasizes the need for modular platforms that allow tailored biological complexity.
Custom model & assay
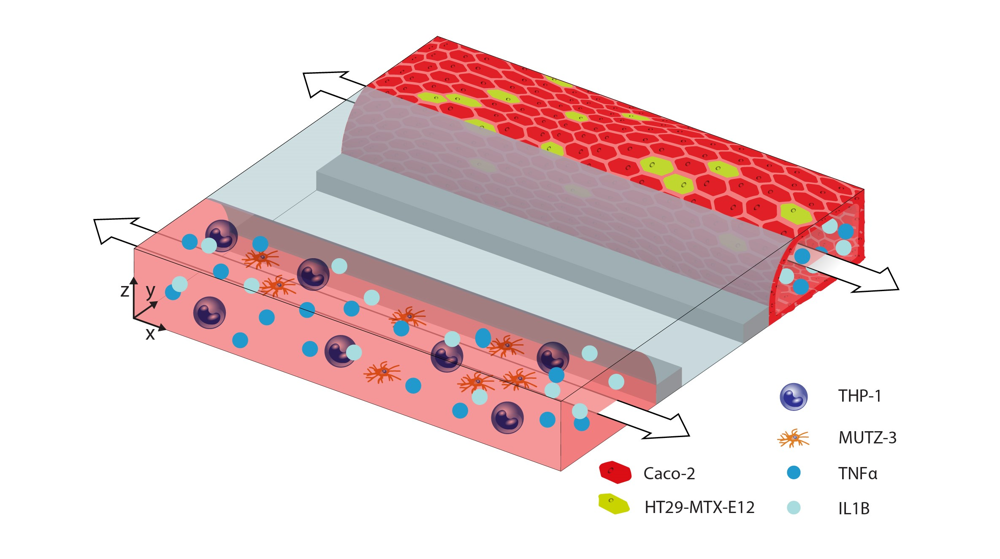
Together with Philip Morris International (PMI) we developed a 3D in vitro intestinal model consisting of epithelial and immune-competent cells. We demonstrated that intestinal inflammation can be induced or prevented by specific compounds, as assessed by changes in barrier function and cytokine release.
Finally, we showed how the biological complexity of the model can be adjusted, allowing us to optimize the combination of biological components relevant to intestinal inflammation and the specific research question.
Key results
- An intestine-on-a-chip model of which the biological complexity can be tailored to the specific research question: plug-and-play modularity
- Induction and prevention of intestinal inflammation
- Suitable for studying intestinal inflammation or screening anti-inflammatory compounds
Want to know more?
Get up to speed with 3D tissue culture and learn how OrganoPlate® supports your research needs.
