 Gastrointestinal toxicity (GIT) is a common side effect of cancer therapies and other treatments, often leading to dose-limiting complications. Traditional toxicity models fail to replicate the human gut environment, limiting their predictive power.
Gastrointestinal toxicity (GIT) is a common side effect of cancer therapies and other treatments, often leading to dose-limiting complications. Traditional toxicity models fail to replicate the human gut environment, limiting their predictive power.
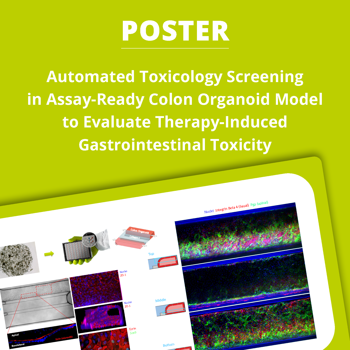
MIMETAS' ready-to-use 3D Colon Organoid model overcomes these limitations by providing a scalable, perfused, and assay-ready microfluidic system that closely mimics human intestinal physiology. This platform enables high-throughput toxicology screening, allowing for accurate evaluation of acute and chronic drug-induced GI toxicity.
Key Features of the Model
- 3D human colon organoid tubules – Replicates native intestinal architecture
- Automated, high-throughput testing – Supports 40-64 tubules per plate
- Perfusable & membrane-free – Enables apical and basolateral drug exposure
- Barrier integrity assessment – TEER & viability assays for mechanistic toxicity insights
- Compatible with standard lab equipment – Integrates with liquid handling & fluorescence imaging
Download the poster to see how this model enhances drug safety testing.
