 Leiden, Netherlands, February 17, 2025 – A new study published in Toxins presents a multi-parametric approach to assess barrier disruption in advanced 3D human gut models. The research uses candidalysin, a cytolytic peptide toxin produced by Candida albicans, as a model compound to investigate epithelial damage. Candidalysin is known to compromise barrier integrity and trigger immune responses during fungal infections. By integrating multiple complementary assays, MIMETAS researchers established a systematic approach for evaluating gut barrier function.
Leiden, Netherlands, February 17, 2025 – A new study published in Toxins presents a multi-parametric approach to assess barrier disruption in advanced 3D human gut models. The research uses candidalysin, a cytolytic peptide toxin produced by Candida albicans, as a model compound to investigate epithelial damage. Candidalysin is known to compromise barrier integrity and trigger immune responses during fungal infections. By integrating multiple complementary assays, MIMETAS researchers established a systematic approach for evaluating gut barrier function.
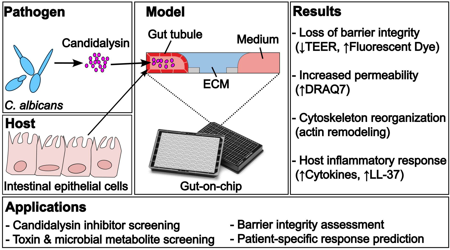
Key Findings: Barrier Disruption and Inflammation
The study revealed that candidalysin significantly compromises epithelial barrier integrity, as evidenced by:
- Reduced trans-epithelial electrical resistance (TEER), indicating impaired cell junctions.
- Increased permeability, shown by leakage of fluorescent molecules through the epithelial layer.
- Actin cytoskeleton remodeling, a key sign of cellular stress and structural changes.
- Elevated secretion of inflammatory markers, including cytokines and antimicrobial peptides, indicating an immune response to toxin exposure.
A proof-of-concept experiment using patient-derived intestinal organoid tubules further validated key aspects of the barrier impairment observed in Caco-2 models.
Implications for Drug Development and Screening
This systematic approach to evaluating barrier function provides researchers with robust, quantitative tools for drug development and toxicology studies. The study used two commercially available models from MIMETAS - the OrganoReady® Colon Caco-2 and OrganoReady® Colon Organoid platforms. This combination of standardized models with multiple quantitative readouts offers practical solutions for researchers evaluating drug candidates affecting epithelial integrity, whether as desired therapeutic effects or potential safety concerns.
