Leiden, January 5, 2023 - Scientists from MIMETAS and the University of Texas Health Center develop a model system that supports the culture of clinically relevant PDX cells that recapitulate aspects of the prostate cancer (PCa) tumor microenvironment (TME).
 Advancements in cancer models have paved the way for revised and improved systems, such as patient-derived xenografts (PDX), that better replicate the human condition. PDXs are developed through patient tumor tissue implantation and serial propagation in murine hosts and as such, closely maintain parental tumor morphology, heterogeneity, gene expression, and drug response. This addresses the lack of translatability seen between traditional monolayer cultures used for high throughput screening (HTS) in developing anti-cancer compounds, and tumor cells in situ morphology.
Advancements in cancer models have paved the way for revised and improved systems, such as patient-derived xenografts (PDX), that better replicate the human condition. PDXs are developed through patient tumor tissue implantation and serial propagation in murine hosts and as such, closely maintain parental tumor morphology, heterogeneity, gene expression, and drug response. This addresses the lack of translatability seen between traditional monolayer cultures used for high throughput screening (HTS) in developing anti-cancer compounds, and tumor cells in situ morphology.
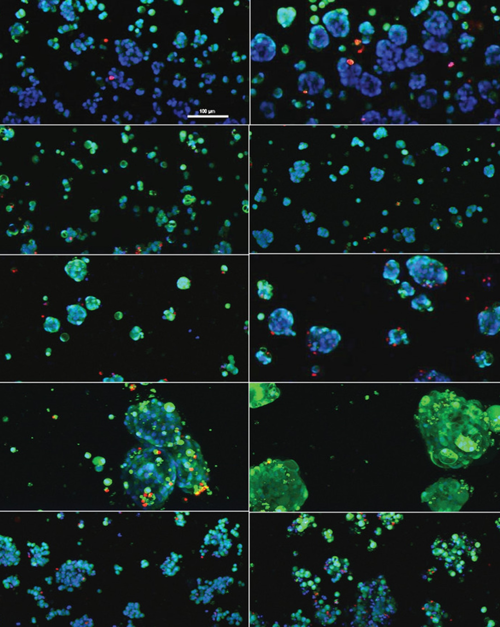
Whilst recent PDX-based clinical trials have improved the predictability of laboratory drug assays toward human use, moving to scaled up exploratory studies prove difficult due to time-, labor- and cost-intensity of incorporating murine models. Complex multilineage co-cultures of the TME also further complicate ex vivo implementation. When looking at prostate cancer in particular, applications of PDX models are scarce and most likely due to poor take rate. As such, there is a need for platforms that enable the use of PDX-derived tissue, and in particular of PCa-derived models. Recent developments in three-dimensional (3D) in vitro cell culture models have shown promise in bridging the gap between in vivo and traditional in vitro cell cultures, allowing the incorporation of microenvironmental cues essential in tumorigenesis, metastasis, treatment response, and other aspects of cancer biology.
To this end, scientists used the OrganoPlate®, a state-of-the-art microfluidics-based perfusion-enabled device, to develop multiple PCa-derived PDX models as 3D clusters within engineered biomimetic hydrogel matrices, alongside bone marrow-derived stromal cells and a perfused endothelial channel. Polymeric hydrogel matrices were customized for each cell type, enabling cell survival in vitro and facile imaging across all conditions. PCa PDXs showed unique morphologies and interactions with TME partners, retention of known phenotype, and expected response to standard PCa therapeutics.
The results presented in the article provide a paradigm that enables in vitro use of PCa PDXs in a high-throughput compatible format, which has not been accessible previously.
