About the research
Angiogenesis plays a fundamental role in both health and disease. The process of angiogenesis and formation of microvasculature is crucial to study the onset and progression of many diseases, including cancer, macular degeneration, Alzheimer’s disease, vascular fibrosis, and inflammation.
For the discovery of new drug targets that target the angiogenesis process, drug research heavily relies on in vitro models. Currently used in vitro angiogenesis models have limited translatability to the in vivo situation. To advance pre-clinical vascular drug research there is a need for in vitro assays that closely mimic the process of angiogenesis in vivo. To enable drug screening, physiologically relevant culture conditions are required which capture both robustness and scalability.
Together with researchers from LUMC, Leiden University, and Ncardia, we developed a phenotypic angiogenesis inhibition assay in the OrganoPlate® platform.
Phenotypic inhibition assay
The developed angiogenesis inhibition assay includes endothelial cells (ECs) from induced pluripotent stem cells (iPSC). In total 40 individually addressable and perfused microvessels were developed, including physiologically relevant cues such as a three-dimensional hydrogel, flow, and angiogenic gradients.
Identical sprouting behavior observed
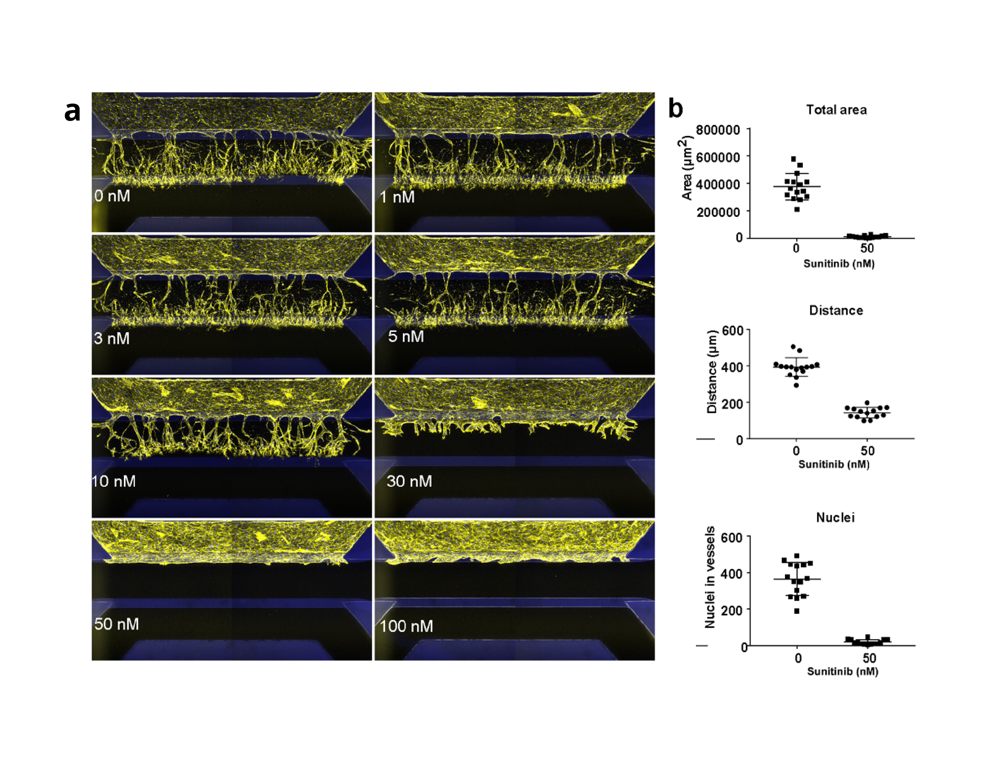
To assess its performance and suitability for anti-angiogenic drug screening, the group compared sprouting behavior including tip formation, directional sprouting, and lumen formation to primary ECs behavior. By objecting the microvessels to sunitinib (VEGF receptor type 2 inhibitor) and a transient glycolysis inhibitor (3PO), sprouting of both primary- and iPSC-ECs significantly reduced. The assay shows its robustness, reproducibility and its potential to be integrated within a drug-screening infrastructure as the assay is amenable to screening of anti-angiogenic compounds.
Key results
- In vitro assay includes relevant physiological cues: 3D hydrogel, flow, angiogenic gradients
- Identical sprouting behavior observed in both ECs and iPSC cultures
- Stable sprout formation with lumen, tip stalk cells, neovessel perfusion, and anastomosis
- Robust, and scalable angiogenesis inhibition assay. Suitable for screening of anti-angiogenic compounds
Want to know more?
Get up to speed with 3D tissue culture and learn how OrganoPlate® supports your research needs.
