Building a High-throughput and Automated Liver-on-a-chip Model for Hepatotoxicity Detection
Drug induced liver injury (DILI) is the leading cause of approved drug withdrawal from the market and presents a major health concern as more than 50% of acute liver failures are caused by DILI. Identification of hepatotoxic compounds in the preclinical phase of drug development is key to preventing DILI, however currently employed animal and two-dimensional (2D) in vitro models often fail to predict clinical hepatotoxicity.Physiologically relevant and efficient in vitro models that mimic the human liver microenvironment and clinical outcome are necessary. Microphysiological system (MPS) models of the liver, or liver-on-a-chip models, incorporate principal characteristics of the in vivo liver such as 3D structure, co-culture, and perfusion. However, many MPS liver models require complex assembly and therefore are low- throughput, limiting their use in a drug screening capacity.In this application note, we demonstrate the development and assessment of a 3D microfluidic liver-on-a-chip model in the OrganoPlate®, ready to be used for high-throughput hepatotoxicity screening: the OrganoPlate LiverToxTM.Benefits of the model:A robust and validated liver-on-a-chip model to use for high-throughput hepatotoxicity screeningRecapitulate the in vivo liver through a 3D three liver-cell type model, without the use of animalsPerform a compound library screen to assess, rank, and prioritize compounds for follow-up mechanistic studies
Building a High-throughput and Automated Liver-on-a-chip Model for Hepatotoxicity Detection

Building a High-throughput and Automated Liver-on-a-chip Model for Hepatotoxicity Detection
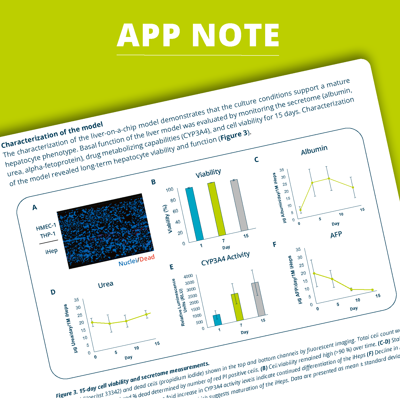
The OrganoPlate® 2-lane 96 is a microfluidic cell culture platform that enables culturing and screening of a range of miniaturized 3D organs and tissue models. Here we describe the development of a functional 3D liver-on-a-chip model on the OrganoPlate®. The 3-liver cell type model was constructed by seeding differentiated induced pluripotent stem-cell derived hepatocytes (iHeps) in collagen extracellular matrix in the top channel of the chip, and endothelial and Kupffer cells in the bottom channel. Characterization of the model showed that the culture achieved a mature hepatocyte phenotype over time. The model was capable of detecting known hepatotoxins and four robust assays were identified and used in a compound library screen of 159 compounds. Identified toxic hits were ranked in hepatotoxicity by a composite score and a follow-up dose-response assessment was conducted. The platform’s compatibility with standard laboratory equipment and automation makes this liver-on-a-chip model amenable to highthroughput screening. This physiologically relevant model enables hepatotoxicity testing and provides valuable insight into drug efficacy studies for liver diseases.

Building a High-throughput and Automated Liver-on-a-chip Model for Hepatotoxicity Detection
The OrganoPlate® 2-lane 96 is a microfluidic cell culture platform that enables culturing and screening of a range of miniaturized 3D organs and tissue models. Here we describe the development of a functional 3D liver-on-a-chip model on the OrganoPlate®. The 3-liver cell type model was constructed by seeding differentiated induced pluripotent stem-cell derived hepatocytes (iHeps) in collagen extracellular matrix in the top channel of the chip, and endothelial and Kupffer cells in the bottom channel. Characterization of the model showed that the culture achieved a mature hepatocyte phenotype over time. The model was capable of detecting known hepatotoxins and four robust assays were identified and used in a compound library screen of 159 compounds. Identified toxic hits were ranked in hepatotoxicity by a composite score and a follow-up dose-response assessment was conducted. The platform’s compatibility with standard laboratory equipment and automation makes this liver-on-a-chip model amenable to highthroughput screening. This physiologically relevant model enables hepatotoxicity testing and provides valuable insight into drug efficacy studies for liver diseases.

Building a High-throughput and Automated Liver-on-a-chip Model for Hepatotoxicity Detection
The OrganoPlate® 2-lane 96 is a microfluidic cell culture platform that enables culturing and screening of a range of miniaturized 3D organs and tissue models. Here we describe the development of a functional 3D liver-on-a-chip model on the OrganoPlate®. The 3-liver cell type model was constructed by seeding differentiated induced pluripotent stem-cell derived hepatocytes (iHeps) in collagen extracellular matrix in the top channel of the chip, and endothelial and Kupffer cells in the bottom channel. Characterization of the model showed that the culture achieved a mature hepatocyte phenotype over time. The model was capable of detecting known hepatotoxins and four robust assays were identified and used in a compound library screen of 159 compounds. Identified toxic hits were ranked in hepatotoxicity by a composite score and a follow-up dose-response assessment was conducted. The platform’s compatibility with standard laboratory equipment and automation makes this liver-on-a-chip model amenable to highthroughput screening. This physiologically relevant model enables hepatotoxicity testing and provides valuable insight into drug efficacy studies for liver diseases.

Building a High-throughput and Automated Liver-on-a-chip Model for Hepatotoxicity Detection
The OrganoPlate® 2-lane 96 is a microfluidic cell culture platform that enables culturing and screening of a range of miniaturized 3D organs and tissue models. Here we describe the development of a functional 3D liver-on-a-chip model on the OrganoPlate®. The 3-liver cell type model was constructed by seeding differentiated induced pluripotent stem-cell derived hepatocytes (iHeps) in collagen extracellular matrix in the top channel of the chip, and endothelial and Kupffer cells in the bottom channel. Characterization of the model showed that the culture achieved a mature hepatocyte phenotype over time. The model was capable of detecting known hepatotoxins and four robust assays were identified and used in a compound library screen of 159 compounds. Identified toxic hits were ranked in hepatotoxicity by a composite score and a follow-up dose-response assessment was conducted. The platform’s compatibility with standard laboratory equipment and automation makes this liver-on-a-chip model amenable to highthroughput screening. This physiologically relevant model enables hepatotoxicity testing and provides valuable insight into drug efficacy studies for liver diseases.

Building a High-throughput and Automated Liver-on-a-chip Model for Hepatotoxicity Detection
The OrganoPlate® 2-lane 96 is a microfluidic cell culture platform that enables culturing and screening of a range of miniaturized 3D organs and tissue models. Here we describe the development of a functional 3D liver-on-a-chip model on the OrganoPlate®. The 3-liver cell type model was constructed by seeding differentiated induced pluripotent stem-cell derived hepatocytes (iHeps) in collagen extracellular matrix in the top channel of the chip, and endothelial and Kupffer cells in the bottom channel. Characterization of the model showed that the culture achieved a mature hepatocyte phenotype over time. The model was capable of detecting known hepatotoxins and four robust assays were identified and used in a compound library screen of 159 compounds. Identified toxic hits were ranked in hepatotoxicity by a composite score and a follow-up dose-response assessment was conducted. The platform’s compatibility with standard laboratory equipment and automation makes this liver-on-a-chip model amenable to highthroughput screening. This physiologically relevant model enables hepatotoxicity testing and provides valuable insight into drug efficacy studies for liver diseases.

Building a High-throughput and Automated Liver-on-a-chip Model for Hepatotoxicity Detection
The OrganoPlate® 2-lane 96 is a microfluidic cell culture platform that enables culturing and screening of a range of miniaturized 3D organs and tissue models. Here we describe the development of a functional 3D liver-on-a-chip model on the OrganoPlate®. The 3-liver cell type model was constructed by seeding differentiated induced pluripotent stem-cell derived hepatocytes (iHeps) in collagen extracellular matrix in the top channel of the chip, and endothelial and Kupffer cells in the bottom channel. Characterization of the model showed that the culture achieved a mature hepatocyte phenotype over time. The model was capable of detecting known hepatotoxins and four robust assays were identified and used in a compound library screen of 159 compounds. Identified toxic hits were ranked in hepatotoxicity by a composite score and a follow-up dose-response assessment was conducted. The platform’s compatibility with standard laboratory equipment and automation makes this liver-on-a-chip model amenable to highthroughput screening. This physiologically relevant model enables hepatotoxicity testing and provides valuable insight into drug efficacy studies for liver diseases.

Building a High-throughput and Automated Liver-on-a-chip Model for Hepatotoxicity Detection
The OrganoPlate® 2-lane 96 is a microfluidic cell culture platform that enables culturing and screening of a range of miniaturized 3D organs and tissue models. Here we describe the development of a functional 3D liver-on-a-chip model on the OrganoPlate®. The 3-liver cell type model was constructed by seeding differentiated induced pluripotent stem-cell derived hepatocytes (iHeps) in collagen extracellular matrix in the top channel of the chip, and endothelial and Kupffer cells in the bottom channel. Characterization of the model showed that the culture achieved a mature hepatocyte phenotype over time. The model was capable of detecting known hepatotoxins and four robust assays were identified and used in a compound library screen of 159 compounds. Identified toxic hits were ranked in hepatotoxicity by a composite score and a follow-up dose-response assessment was conducted. The platform’s compatibility with standard laboratory equipment and automation makes this liver-on-a-chip model amenable to highthroughput screening. This physiologically relevant model enables hepatotoxicity testing and provides valuable insight into drug efficacy studies for liver diseases.

Building a High-throughput and Automated Liver-on-a-chip Model for Hepatotoxicity Detection
The OrganoPlate® 2-lane 96 is a microfluidic cell culture platform that enables culturing and screening of a range of miniaturized 3D organs and tissue models. Here we describe the development of a functional 3D liver-on-a-chip model on the OrganoPlate®. The 3-liver cell type model was constructed by seeding differentiated induced pluripotent stem-cell derived hepatocytes (iHeps) in collagen extracellular matrix in the top channel of the chip, and endothelial and Kupffer cells in the bottom channel. Characterization of the model showed that the culture achieved a mature hepatocyte phenotype over time. The model was capable of detecting known hepatotoxins and four robust assays were identified and used in a compound library screen of 159 compounds. Identified toxic hits were ranked in hepatotoxicity by a composite score and a follow-up dose-response assessment was conducted. The platform’s compatibility with standard laboratory equipment and automation makes this liver-on-a-chip model amenable to highthroughput screening. This physiologically relevant model enables hepatotoxicity testing and provides valuable insight into drug efficacy studies for liver diseases.

