About the research
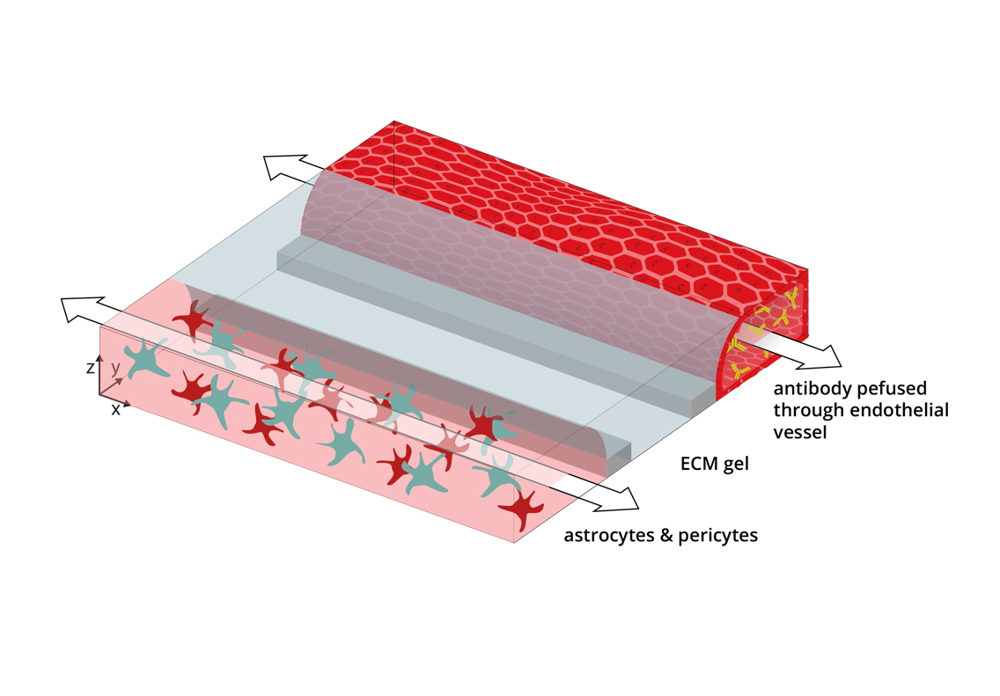
The brain vasculature is made up of specialized endothelial cells that form a tight blood-brain barrier (BBB). This barrier prevents many drugs as well as large biological molecules such as antibodies, from passively diffusing into the brain. For this reason, specialized techniques need to be developed to improve drug targeting to the brain. One of these techniques is receptor mediated transcytosis (RMT) of antibodies. This entails the design of a therapeutic antibody that binds to a receptor present on the brain endothelial cells, such as the transferrin receptor (TfR), thus improving its targeting to the brain. After binding to the TfR on the apical side of the brain endothelial cells, the antibody is transcytosed and subsequently released on the basolateral side, i.e. the brain side.
In vitro models of the blood-brain barrier are of great value for evaluating, for example, the efficiency of antibody transcytosis across the BBB and improve the treatment of neurodegenerative diseases.
Custom model & assay
We developed an in vitro BBB-on-a-chip model comprising human brain endothelial cells, astrocytes, and pericytes. The endothelial cells formed a functional barrier that is able to retain fluorescent dyes and antibodies, and that prevent antibodies from leaking out of the model through passive mechanisms.
Perfusion with a target antibody
The model was perfused with a target antibody, designed to cross the BBB via targeting of the TfR. Next to this, we perfused the model with a control antibody that doesn’t bind to human cells and, therefore is not supposed to cross.
We found an approximately twofold higher passage rate of the target antibody compared to the control antibody, indicating that this model is indeed suitable for the evaluation of BBB shuttle technologies.
Key results
- Unique 3D and membrane-free BBB-on-a-chip model compromising human brain endothelial cells, astrocytes, and pericytes
- Formation of a functional barrier able to retain fluorescent dyes and antibodies
- First BBB in vitro model that includes flow and can study barrier function and antibody transcytosis in high-throughput
Want to know more?
Get up to speed with 3D tissue culture and learn how OrganoPlate® supports your research needs.
