As the formation and remodeling of new blood vessels play a critical role in health (e.g. wound healing, embryonic development) and disease (e.g. cancer), understanding its precise mechanisms are of significant interest. Many anti-angiogenic drugs have been developed to complement standard chemotherapies in cancer therapy, while pro-angiogenic small molecules may hold potential in regenerative applications.1,2 Maximizing these therapeutic approaches requires suitable in vitro models and an understanding of the differences between the two fundamental processes by which new blood vessels are formed.
in health (e.g. wound healing, embryonic development) and disease (e.g. cancer), understanding its precise mechanisms are of significant interest. Many anti-angiogenic drugs have been developed to complement standard chemotherapies in cancer therapy, while pro-angiogenic small molecules may hold potential in regenerative applications.1,2 Maximizing these therapeutic approaches requires suitable in vitro models and an understanding of the differences between the two fundamental processes by which new blood vessels are formed.
In this article, we explain the difference between angiogenesis and vasculogenesis and shine a spotlight on how angiogenesis is modeled in current systems.
What is angiogenesis?
Angiogenesis is the formation and remodeling of new blood vessels and capillaries from the growth of pre-existing blood vessels.3 This can be achieved through endothelial sprouting or intussusceptive (splitting) microvascular growth.
of new blood vessels and capillaries from the growth of pre-existing blood vessels.3 This can be achieved through endothelial sprouting or intussusceptive (splitting) microvascular growth.
The key cellular events driving angiogenesis are:4,5
- Extracellular matrix (ECM) degradation in response to angiogenic stimuli
- Migration: Tip cells migrate in response to growth factor gradients
- Proliferation: Stalk cells proliferate and extend the sprout which has formed off the existing vasculature
Endothelial cells undergo these key processes to create a tube, containing a lumen – a dynamic tunnel space that propels blood flow and facilitates the exchange of oxygen, carbon dioxide, nitric oxide, and nutrients.6
Angiogenic growth also supports the invasion of tumor cells in healthy tissue and is commonly measured in cancer research.7
What is vasculogenesis?
Vasculogenesis is the de novo synthesis of blood vessels. Vasculogenesis is defined as the differentiation of precursor cells (angioblasts) into endothelial cells and the de novo formation of a primitive vascular network.8
When do angiogenesis and vasculogenesis occur?
Vasculogenesis is an important process during early embryonic development, in which cells in the mesoderm are induced to form primitive endothelial cells and a primary vascular network.9 Angiogenesis enables remodeling and maturation of this network, which involves new blood vessels splitting or sprouting from the existing vessels.
In adult life, angiogenesis is a key part of common physiological processes such as wound healing and various stages of the female reproductive cycle (e.g. endometrial and ovarian follicular growth).10-12 Excessive angiogenesis feeds diseased tissue and contributes to the pathogenesis of cancer, psoriasis, AIDS complications, rheumatoid arthritis, and blindness associated with diabetic retinopathy.13-16 On the other hand, insufficient angiogenesis is a characteristic of many common disorders including stroke and coronary heart disease.17
The role of vasculogenesis in the mature adult is less clear. Initially, it was thought that vasculogenesis was limited to embryonic development, however the identification of endothelial precursor cells in adult bone marrow and peripheral blood suggests that vasculogenesis may also occur in adults.18 Several studies have supported this possibility; in animal models of ischemia it was shown that endothelial precursor cells are recruited to neovascularization sites.19 However, the extent of the involvement of these cells in vascularization is unclear.
A report of the ability of aggressive cancer cells to form de novo vascular networks (termed “vascular mimicry”) and acquire the characteristics of endothelial cells sparked a long debate regarding postnatal vasculogenesis.20,21 The term “vascular mimicry” is widely (and controversially) used in the literature to refer to the tumor blood supply system that takes place independently of angiogenesis of endothelial cells, associated with a poor outcome in cancer patients.22 Meanwhile, others have raised concerns about vascular mimicry research and call for a standard classification regarding its criteria and modeling.23,24
This confusion and controversy is partly due to the difficulty that lies in studying vascular growth in vivo and the lack of suitable three-dimensional models available for research.22,24
How has angiogenesis traditionally been modeled in vitro?
In vitro experiments to date have modeled key aspects of angiogenic mechanisms and endothelial cell biology using scratch assays, Boyden chambers, and tube formation assays in various formats.
Scratch assay
The in vitro scratch assay, also known as a “wound healing assay”, 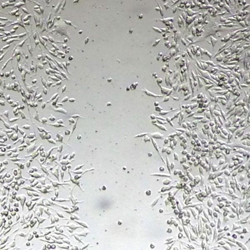 is used to measure cell migration in two dimensions. Once a “scratch” has been introduced to cells grown in a monolayer, imaging technology is used to quantify the migration rate of cells that move to close the gap.25 The scratch assay is a straightforward and low-cost method that can be used to study the effect of angiogenic and anti-angiogenic factors on individual and homogeneous cell populations. Its limitations include:
is used to measure cell migration in two dimensions. Once a “scratch” has been introduced to cells grown in a monolayer, imaging technology is used to quantify the migration rate of cells that move to close the gap.25 The scratch assay is a straightforward and low-cost method that can be used to study the effect of angiogenic and anti-angiogenic factors on individual and homogeneous cell populations. Its limitations include:
- Only one key aspect of angiogenesis is modeled, migration
- The absence of a chemical gradient makes it difficult to distinguish directional (chemotaxis) from random (chemokinesis) migration
- Migration can be mistaken for proliferation, unless the latter is completely blocked
- Two-dimensional migration and hard substrate limits physiological relevance
- Relatively large amounts of cells and chemicals are required
- Time consuming (1-2 days for cell monolayer formation, 8-18 hours for cell migration to close the scratch)
Boyden chamber
The Boyden chamber migration assay, also known as the trans-well migration assay, is used for studies of cell migration and invasion. It consists of two medium-filled chambers separated by a porous membrane and assesses the number of cells that migrate from the upper side to the lower side of the membrane.26 Cells are seeded in the top of a cylindrical cell culture insert, which is positioned inside the well of a cell culture plate. Cells migrate through pores in the insert, towards a chemoattractant positioned below. While the Boyden chamber has been used to generate insights into the chemotactic migratory response of endothelial cells to various angiogenic inducers and inhibitors, it does have many limitations, listed below:27
- ECM is often lacking; ECM degradation cannot occur
- Only measures cell migration/invasion; not a complete model
- There is no lumen formation
- The gradient equalizes relatively quickly, limiting prolonged studies
- Time-consuming; cell counting is manual and dependent on the operator’s expertise
- Commercial inserts can be expensive
Tube formation assay
Tube formation assays have been used to assess the effect of various genes and pathways in angiogenesis.28 They can be conducted with relative speed and ease, in 96-well plates and in 15-well slide formats. Endothelial cells (typically human umbilical vein endothelial cells, HUVECs) are typically used for this assay, where they are pipetted as single cells in suspension into an ECM of basement membrane-like substrate in each well. Initially, cells attach to the matrix, before migrating towards each other and aggregating in a tube-like shape, which can be assessed by measuring the tube area, length, or number of branch points. Traditionally, this has been the preferred method for assessing angiogenic regulators.29
However, the tube formation assay does not truly model angiogenesis, partly because there are no pre-existing vessels – a key feature of the angiogenic process. By definition, the tube formation assay more closely models vasculogenesis – the de novo formation of a vascular network from endothelial cells. As tube formation assays begin with a single cell suspension, and the resulting vessel networks don’t necessarily retain angiogenic “sprouting” potential, this assay format is more representative of vasculogenesis than angiogenesis. While the endothelial cells do aggregate and form networks, the tube-like formations can be devoid of a lumen, another critical characteristic of angiogenesis.
What is needed to model angiogenesis in vitro?
To truly model angiogenesis, in vitro models would incorporate those elements that are central to the definition of angiogenesis, including the presence of pre-existing blood vessels and lumen formation. The formation and remodeling of new blood vessels and capillaries from pre-existing blood vessels is what separates angiogenesis from vasculogenesis (de novo vessel formation).
elements that are central to the definition of angiogenesis, including the presence of pre-existing blood vessels and lumen formation. The formation and remodeling of new blood vessels and capillaries from pre-existing blood vessels is what separates angiogenesis from vasculogenesis (de novo vessel formation).
The formation of endothelial cell lumen, i.e. the dynamic tunnel space that enables blood to pass through the vessel, is a critical part of normal physiology that should be accounted for. Many in vitro angiogenesis models have failed to demonstrate lumen formation, which is not synonymous with the aggregation of cells in a tube-like shape. Perfusion of newly formed vessels would verify lumen formation in in vitro models and provide an important survival and stabilizing factor for the vessels, adding a more physiologically relevant dimension.30
In addition to lumen formation and pre-existing vessels, an ideal in vitro platform would incorporate all steps of angiogenesis that occur in vivo. This includes the presence of a growth factor gradient and a three-dimensional environment resulting in multi-dimensional sprouting,30 while increased stability of newly-grown microvessels would enable experiments of longer duration. Models which allow multiple cell types would provide opportunities for further studies of the tumor microenvironment and the role of immune cells in angiogenesis.30 The ability to assess different aspects of cell behavior (i.e. ECM degradation, migration, and proliferation) while verifying lumen formation on the same platform would also improve the translation of findings from in vitro models to in vivo situations.
Angiogenesis research requires more physiologically relevant models
Given the strong therapeutic potential of angiogenic and anti-angiogenic compounds, there has been a strong research interest in angiogenesis and the development of appropriate in vitro models. While imperfect models can be valuable for answering specific research questions, it is important to recognize their limitations. By definition, the term “angiogenesis” refers to microvessel formation from pre-existing vessels. Current models are not true representations of angiogenesis, and some instead model the de novo synthesis of vascular networks that are devoid of lumen formation. More physiologically relevant models are needed to explore the molecular mechanisms of angiogenesis, and this article has highlighted several fundamental features that should be incorporated.
Don't miss our next blog post where we show you how modern in vitro angiogenesis models have evolved to better represent the processes described here.
References
- Comunanza, V., & Bussolino, F. (2017). Therapy for Cancer: Strategy of Combining Anti-Angiogenic and Target Therapies. Frontiers in Cell and Developmental Biology, 5, 10 https://doi.org/10.3389/fcell.2017.00101
- Das, A., Merrill, P., Wilson, J., Turner, T., Paige, M., Capitosti, S., Brown, M., Freshcorn, B., Sok, M. C. P., Song, H., & Botchwey, E. A. (2019). Evaluating Angiogenic Potential of Small Molecules Using Genetic Network Approaches. Regenerative Engineering and Translational Medicine, 5(1), 30–41. https://doi.org/10.1007/s40883-018-0077-8
- Risau, W. (1997). Mechanisms of angiogenesis. Nature, 386(6626), 671–674. https://doi.org/10.1038/386671a0
- Norton, K.-A., & Popel, A. S. (2016). Effects of endothelial cell proliferation and migration rates in a computational model of sprouting angiogenesis. Scientific Reports, 6(1), 36992. https://doi.org/10.1038/srep36992
- Neve, A., Cantatore, F. P., Maruotti, N., Corrado, A., & Ribatti, D. (2014). Extracellular Matrix Modulates Angiogenesis in Physiological and Pathological Conditions. BioMed Research International, 2014, 1–10. https://doi.org/10.1155/2014/756078
- Davis, G. E., Stratman, A. N., Sacharidou, A., & Koh, W. (2011). Molecular Basis for Endothelial Lumen Formation and Tubulogenesis During Vasculogenesis and Angiogenic Sprouting. In International Review of Cell and Molecular Biology (Vol. 288, pp. 101–165). Elsevier. https://doi.org/10.1016/B978-0-12-386041-5.00003-0
- Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of Cancer: The Next Generation. Cell, 144(5), 646–674. https://doi.org/10.1016/j.cell.2011.02.013
- Risau, W., & Flamme, I. (1995). Vasculogenesis. Annual Review of Cell and Developmental Biology, 11(1), 73–91. https://doi.org/10.1146/annurev.cb.11.110195.000445
- Goldie, L. C., Nix, M. K., & Hirschi, K. K. (2008). Embryonic vasculogenesis and hematopoietic specification. Organogenesis, 4(4), 257–263. https://doi.org/10.4161/org.4.4.7416
- DiPietro, L. A. (2016). Angiogenesis and wound repair: When enough is enough. Journal of Leukocyte Biology, 100(5), 979–984. https://doi.org/1189/jlb.4MR0316-102R
- Hazzard, T. M., & Stouffer, R. L. (2000). Angiogenesis in ovarian follicular and luteal development. Best Practice & Research Clinical Obstetrics & Gynaecology, 14(6), 883–900. https://doi.org/10.1053/beog.2000.0133
- Demir, R., Yaba, A., & Huppertz, B. (n.d.). Vasculogenesis and angiogenesis in the endometrium during menstrual cycle and implantation—ScienceDirect. Acta Histochemica, 112(3), 203–214
- Richarz, N. A., Boada, A., & Carrascosa, J. M. (2017). Angiogenesis in Dermatology – Insights of Molecular Mechanisms and Latest Developments. Actas Dermo-Sifiliográficas, 108(6), 515–523. https://doi.org/10.1016/j.ad.2016.12.001
- Basta, D., Latinovic, O., Lafferty, M. K., Sun, L., Bryant, J., Lu, W., Caccuri, F., Caruso, A., Gallo, R., & Garzino-Demo, A. (2015). Angiogenic, lymphangiogenic and adipogenic effects of HIV-1 matrix protein p17. Pathogens and Disease, 73(8), ftv062. https://doi.org/10.1093/femspd/ftv062
- Elshabrawy, H. A., Chen, Z., Volin, M. V., Ravella, S., Virupannavar, S., & Shahrara, S. (2015). The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis, 18(4), 433–448. https://doi.org/10.1007/s10456-015-9477-2
- Crawford, T., Alfaro III, D., Kerrison, J., & Jablon, E. (2009). Diabetic Retinopathy and Angiogenesis. Current Diabetes Reviews, 5(1), 8–13. https://doi.org/10.2174/157339909787314149
- DeWitt, N. (2005). Angiogenesis. Nature, 438(7070), 931–931. https://doi.org/10.1038/438931a
- Drake, C. J. (2003). Embryonic and adult vasculogenesis. Birth Defects Research Part C: Embryo Today: Reviews, 69(1), 73–82. https://doi.org/10.1002/bdrc.10003
- Asahara, T., Murohara, T., Sullivan, A., Silver, M., van der Zee, R., Li, T., Witzenbichler, B., Schatteman, G., & Isner, J. M. (1997). Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science, 275(5302), 964–966. https://doi.org/10.1126/science.275.5302.964
- Maniotis, A. J., Folberg, R., Hess, A., Seftor, E. A., Gardner, L. M. G., Pe’er, J., Trent, J. M., Meltzer, P. S., & Hendrix, M. J. C. (1999). Vascular Channel Formation by Human Melanoma Cells in Vivo and in Vitro: Vasculogenic Mimicry. The American Journal of Pathology, 155(3), 739–752. https://doi.org/10.1016/S0002-9440(10)65173-5
- Hendrix, M. J. C., Seftor, E. A., Seftor, R. E. B., Chao, J.-T., Chien, D.-S., & Chu, Y.-W. (2016). Tumor cell vascular mimicry: Novel targeting opportunity in melanoma. Pharmacology & Therapeutics, 159, 83–92. https://doi.org/10.1016/j.pharmthera.2016.01.006
- Fernández-Cortés, M., Delgado-Bellido, D., & Oliver, F. J. (2019). Vasculogenic Mimicry: Become an Endothelial Cell “But Not So Much”. Frontiers in Oncology, 9, 803. https://doi.org/10.3389/fonc.2019.00803
- McDonald, D. M., Munn, L., & Jain, R. K. (2000). Vasculogenic Mimicry: How Convincing, How Novel, and How Significant? The American Journal of Pathology, 156(2), 383–388. https://doi.org/10.1016/S0002-9440(10)64740-2
- Valdivia, A., Mingo, G., Aldana, V., Pinto, M., Ramirez, M., Rematal, C., Gonzalez, A., Nualart, F., Corvalan, A., & Owen, G. (n.d.). Frontiers | Fact or Fiction, It Is Time for a Verdict on Vasculogenic Mimicry? | Oncology. Frontiers in Oncology, 9, 680.
- Liang, C.-C., Park, A. Y., & Guan, J.-L. (2007). In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nature Protocols, 2(2), 329–333. https://doi.org/10.1038/nprot.2007.30
- Chen, H.-C. (2004). Boyden Chamber Assay. In J.-L. Guan, Cell Migration (Vol. 294, pp. 015–022). Humana Press. https://doi.org/10.1385/1-59259-860-9:015
- Guy, J.-B., Espenel, S., Vallard, A., Battiston-Montagne, P., Wozny, A.-S., Ardail, D., Alphonse, G., Rancoule, C., Rodriguez-Lafrasse, C., & Magne, N. (2017). Evaluation of the Cell Invasion and Migration Process: A Comparison of the Video Microscope-based Scratch Wound Assay and the Boyden Chamber Assay. Journal of Visualized Experiments, 129, 56337. https://doi.org/10.3791/56337
- DeCicco-Skinner, K. L., Henry, G. H., Cataisson, C., Tabib, T., Gwilliam, J. C., Watson, N. J., Bullwinkle, E. M., Falkenburg, L., O’Neill, R. C., Morin, A., & Wiest, J. S. (2014). Endothelial Cell Tube Formation Assay for the In Vitro Study of Angiogenesis. Journal of Visualized Experiments, 91, 51312. https://doi.org/10.3791/51312
- Arnaoutova, I., & Kleinman, H. K. (2010). In vitro angiogenesis: Endothelial cell tube formation on gelled basement membrane extract. Nature Protocols, 5(4), 628–635. https://doi.org/10.1038/nprot.2010.6
- van Duinen, V., Zhu, D., Ramakers, C., van Zonneveld, A. J., Vulto, P., & Hankemeier, T. (2019). Perfused 3D angiogenic sprouting in a high-throughput in vitro platform. Angiogenesis, 22(1), 157–165. https://doi.org/10.1007/s10456-018-9647-0
Learn more
You might also be interested in:
- [Webinar] Modelling Angiogenesis From Start to Finish in the OrganoPlate®
- [Application note] Perfused 3D angiogenic sprouting in a high-throughput in vitro platform
- [Infographic] Making in vitro 3D Angiogenesis a Reality
- [Video Article] Standardized and Scalable Assay to Study Perfused 3D Angiogenic Sprouting of iPSC-derived Endothelial Cells In Vitro
- [Blog] Dissatisfied With Your Angiogenesis Model? Read This
- [Case Study] Robust angiogenesis inhibition assay in collaboration with Leiden University Medical Center (LUMC)
- [Product] OrganoPlate® 3-lane 40
- Read more about Angiogenesis
